CERTAIN IDENTIFIED INFORMATION HAS BEEN EXCLUDED FROM THIS EXHIBIT BECAUSE IT IS BOTH NOT MATERIAL AND IS THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL
| DARTMOUTH | CONFIDENTIAL |
DARTMOUTH COLLEGE
INTELLECTUAL PROPERTY LICENSE AGREEMENT
This Intellectual Property License Agreement (this "Agreement") by and between the Trustees of Dartmouth College ("Dartmouth") with an address at 11 Rope Ferry Road, Hanover, New Hampshire 03755, and Algernon Pharmaceuticals, Inc., a corporation with principal offices located at 915 - 700 West Pender Street, Vancouver, British Columbia, V6C 1G8, Canada ("Licensee") is effective as of August 6, 2021 (the "Effective Date").
Background
WHEREAS, Dartmouth is an institution of research and higher education and, as a result, has gained certain rights to intellectual property; and
WHEREAS, Dartmouth desires that such intellectual property be developed and utilized to the fullest extent possible so that its benefits can be enjoyed by the general public; and
WHEREAS, to induce Dartmouth to enter into this Agreement and to grant the rights contemplated hereunder, Licensee has agreed under this Agreement to act diligently to develop and commercialize such intellectual property for public use in accordance with the terms and conditions of this Agreement.
NOW, THEREFORE, in consideration of these statements and the mutual promises set forth below, and for other good and valuable consideration, Dartmouth and Licensee hereby agree to the following:
1. Definitions
1.1. "Affiliate" means, with respect to a party, any entity or person that directly or indirectly controls, is controlled by or is under common control with such party. For purposes of this definition, "control" means possession of the power to direct the management of such entity or person, including through ownership of more than fifty percent (50%) of voting securities, governing board voting rights, reserved powers, or by contract.
1.2. "Change of Control" means: (a) any transaction or series of related transactions (including, without limitation, any reorganization, share exchange, consolidation or merger of Licensee or Licensee's parent entity with or into any other entity but excluding any sale of equity securities by the Licensee or Licensee's parent entity undertaken primarily for capital raising purposes) (i) in which the holders of the Licensee's or Licensee's parent entity's outstanding voting equity securities immediately before the first such transaction do not, immediately after any other such transaction, retain stock or other equity interests representing at least fifty percent (50%) of the voting power of the surviving entity of such transaction or (ii) in which at least fifty percent (50%) of the Licensee's or Licensee's parent entity's outstanding voting equity securities are transferred; (b) an initial underwritten public offering of Licensee's or Licensee's parent entity's equity securities under the Securities Act of 1933, as amended; or any alternative transaction such as a reverse merger to become a publicly-traded entity; or (c) a sale, merger, consolidation or other disposition of all or substantially all of the assets of Licensee or Licensee's parent entity.
| DARTMOUTH | CONFIDENTIAL |
1.3. "Confidential Information" means all information disclosed by one party to the other during the negotiation of or under this Agreement in any manner, whether orally, visually or in tangible form, unless such information is subject to an exception described in Section 8.2, provided, however, that Confidential Information that is disclosed in tangible form shall be marked "Confidential" at the time of disclosure and Confidential Information that is disclosed orally or visually shall be identified as confidential at the time of disclosure and subsequently reduced to writing, marked confidential and delivered to the other party within thirty (30) days of such disclosure. Confidential Information shall include, without limitation, materials, know-how and data, technical or non-technical, trade secrets, inventions, methods and processes of a party, whether or not patentable.
1.4. "Derivative" means any revision, enhancement, modification, translation, abridgement, condensation, or expansion that is based on or incorporates any of the Licensed Patents or Licensed Know-How, in whole or in part.
1.5. "Earned Royalty" is defined in Section 5.4.
1.6. "Effective Date" is defined in the Preamble.
1.7. "Field" means Treatment of Human Cancer.
1.8. "First Sale" means mean the first sale to a third party of any Licensed Product in the Licensed Territory.
1.9. IND" means an investigational new drug application filed with the United States Food and Drug Administration prior to beginning clinical trials in humans in the United States.
1.10. "Insolvent" means that (a) an involuntary proceeding shall have been commenced or an involuntary petition shall have been filed seeking (i) liquidation, reorganization or other relief in respect of Licensee or its debts, or of a substantial part of its assets, under any Federal, state or foreign bankruptcy law or (ii) the appointment of a receiver, trustee, custodian, sequestrator, conservator or similar official for Licensee or for a substantial part of its assets, and, in any such case, such proceeding or petition shall continue undismissed for a period of ninety (90) days; (b) Licensee has voluntarily commenced bankruptcy, reorganization, receivership or insolvency proceedings, or any other proceeding under any Federal, state or other law for the relief of debtors; or (c) Licensee is unable to pay its debts as they become due.
1.11. "Inventor" means William G. North.
1.12. "Inventor Agreement" means a consulting or other agreement directly between Licensee and Inventor or (b) an entity controlled by Inventor.
1.13. "License" is defined in Section 2.1.1.
| DARTMOUTH | CONFIDENTIAL |
1.14. "Licensed Know-How" means all formulations, designs, technical information, know- how, show-how, knowledge, data, specifications, test results and other information, whether or not patented or patentable (collectively, "Know-How") which are known, learned, invented or developed by Inventor or Dartmouth as of the Effective Date to the extent that (a) the Know-How is required for the manufacture, use, development, testing, marketing, export, import, offer for sale or sale of any Licensed Product, and (b) Dartmouth possesses the right to license the use of the Know-How to Licensee for commercial purposes.
1.15. "Licensed Patents" means Dartmouth's ownership interest in the United States patents listed in Appendix A, any patent applications claiming priority to such patent applications, together with any continuations, divisionals, and continuations-in-part, to the extent the claims of any such patent or patent application are directed to subject matter specifically described in the patents listed on Appendix A; any reissues, re-examinations, or extensions thereof, or substitutes therefor; and any reissues, re-examinations, or extensions thereof, or substitutes therefor; and the relevant international equivalents of the foregoing.
1.16. "Licensed Product" means either: (a) any method, procedure, service, process or product (including any apparatus or kit) or component part thereof that practice of which or the manufacture, use, sale, import, export or practice of which would, in the absence of the License, infringe (including by contributory or inducement infringement) a Valid Claim of a Licensed Patent (each a "Valid Claim Product"); or (b) any method, procedure, service, process or product that incorporates, uses, is based upon, is derived from, or otherwise is conceived, developed or reduced to practice using any of the Licensed Patents or Licensed Know-How.
1.17. "Licensed Territory" means the United States of America.
1.18. "NDA" or "BLA" means a New Drug Application or a Biologics License Application filed with the U.S. Food and Drug Administration to obtain marketing approval for a Licensed Product in the United States.
1.19. "Net Sales" means the gross amount received from the sale or transfer of the Licensed Products, or from services performed using or constituting Licensed Products by Licensee or its Sublicensees or Affiliates, or any other party authorized by Licensee to sell Licensed Products to third parties, less the following deductions, provided such deductions actually pertain to the disposition of the Licensed Products and are separately invoiced:
(i) all discounts, credits and return allowances, including rebates granted to managed health care or governmental organizations;
(ii) transportation and insurance charges and costs; and
(iii) duties, taxes and other governmental charges levied on the sale, transportation, delivery or practice of Licensed Products, but not including income taxes.
| DARTMOUTH | CONFIDENTIAL |
No deductions shall be made for any other costs or expenses, including but not limited to commissions to independents, agents or those on Licensee's, Sublicensee's or Licensee's Affiliate's payroll or for the cost of collection. Net Sales shall not include the gross invoice price for Licensed Products sold to, or services performed using Licensed Products for, any Affiliate of Licensee unless such Affiliate is an end-user of any Licensed Product, in which case such consideration shall be included in Net Sales at the average selling price charged to a third party during the same quarter.
1.20. "Patent Challenge" means a challenge or opposition to the validity, patentability, enforceability and/or non-infringement of any of the Licensed Patents or otherwise opposing any of the Licensed Patents.
1.21. "Phase I/II Clinical Trial" means a combined Phase I Clinical Trial and Phase II Clinical Trial conducted to evaluate the safety, side effects, and best dose of a drug.
1.22. "Phase II Clinical Trial" means a human clinical trial conducted to evaluate the effectiveness of a drug for a particular indication in patients with a disease and to determine the common short-term side effects and risks associated with the drug as defined in 21 C.F.R §312.21(b) and as practiced according to the standards of the pharmaceutical industry.
1.23. "Phase III Clinical Trial" means expanded controlled and uncontrolled human clinical trials performed after receipt of evidence from Phase II Clinical Trial(s) suggesting the effectiveness of an investigational new drug, as defined by 21 C.F.R §312.21(c), and as practiced according to the standards of the pharmaceutical industry for a Phase III clinical trial and prior to the filing of an NDA or comparable request for marketing approval.
1.24. "Pivotal Trial" means a controlled human clinical trial to evaluate the safety and efficacy of a Licensed Product in which data are sufficient to form the basis for the filing of an NDA or BLA. A Pivotal Trial may not necessarily be a Phase III Clinical Trial.
1.25. "Reasonable Commercial Efforts" means documented efforts that are consistent with those utilized by companies of similar size and type that have successfully developed products and services similar to Licensed Products. In determining Reasonable Commercial Efforts with respect to a particular Licensed Product, Licensee may not reduce such efforts due to the competitive, regulatory or other impact of any other product or method that it owns or licenses, or is developing or commercializing.
1.26. "Sublicense" means the grant by Licensee or Affiliate of Licensee to a third party of a sublicense, cross-license, option, or other right, license, privilege or immunity to practice Licensed Patents or Licensed Know-How or to make, have made, use, sell, have sold, distribute, practice, import or export Licensed Products.
1.27. "Sublicense Income" means consideration in any form received by Licensee or an Affiliate of Licensee in connection with a grant by Licensee or an Affiliate of Licensee to any Sublicensee of a Sublicense, including any license signing fee, license maintenance fee, any fee or other payment pursuant to an option to Sublicense, unearned portion of any minimum royalty payment received by Licensee, distribution or joint marketing fee, research and development funding in excess of Licensee's cost of performing such research and development, any consideration received for an equity interest in or other equity investment in Licensee to the extent such consideration exceeds the fair market value of the equity or other equity investment interest as determined by an independent appraiser mutually agreeable to the parties, the fair market value of any cross-license, and any other consideration of any form, type or kind received by Licensee or any Affiliate of Licensee. Sublicense Income shall also include the amount of any extension of credit or loan to Licensee by such Sublicensee or its affiliate to the extent that such loan is forgiven by such Sublicensee or its affiliate. Notwithstanding any other provision set forth herein, Sublicense Income shall expressly exclude all consideration (a) included within Earned Royalties or Net Sales by any Sublicensee and (b) paid to Licensee or an Affiliate of Licensee or their successors and permitted assigns or their stockholders or members pursuant to a bona fide financing or a Change of Control.
| DARTMOUTH | CONFIDENTIAL |
1.28. "Sublicensee" means any third party that receives a Sublicense from Licensee or any Affiliate of Licensee.
1.29. "Term" is defined in Section 2.4.
1.30. "Valid Claim" means with respect to a particular territory, a pending, or issued and unexpired claim of a Licensed Patent in such territory so long as such claim shall not have been irrevocably abandoned or declared to be invalid in a non-appealable decision of a court or other authority of competent jurisdiction in such territory through no fault or cause of Licensee.
2. License Grant and Term
2.1. Subject to the terms and conditions of this Agreement, Dartmouth hereby grants to Licensee:
2.1.1. An exclusive, royalty-bearing license, subject to the reservation of rights by Dartmouth set forth in Section 2.3, under the Licensed Patents to make, have made, use, develop, sell, have sold, perform, practice, import and export Licensed Products within the Field in the Licensed Territory (the "License").
2.1.2. A non-exclusive, royalty bearing license to use the Licensed Know-How to the extent necessary to make, have made, use, develop, sell, have sold, perform, practice, import and export Licensed Products within the Field in the Licensed Territory.
For clarity, License may, in its sole discretion, sublicense its rights hereunder to an Affiliate of Licensee, and such Affiliate of Licensee shall not be deemed a "Sublicensee".
2.2. To the extent that any invention included within the Licensed Patents has been funded in whole or in part by the United States government, the United States government retains certain rights in such invention as set forth in 35 U.S.C. §200-212 and all regulations promulgated thereunder, as amended, and any successor statutes and regulations (the "Federal Patent Policy"). As a condition of the License granted hereby, Licensee acknowledges and shall and shall cause its Affiliates and Sublicensees to comply with all aspects of the Federal Patent Policy that are applicable to the Licensed Patents, including any obligation that Licensed Products used or sold in the United States be manufactured substantially in the United States. Nothing contained in this Agreement obligates or shall obligate Dartmouth to take any action that would conflict in any respect with its past, current or future obligations to the United States government under the Federal Patent Policy with respect to the Licensed Patents.
| DARTMOUTH | CONFIDENTIAL |
2.3. The License is expressly made subject to Dartmouth's reservation of the right, on behalf of itself and all other non-profit academic research institutions and governmental institutions, to:
(a) make, use and practice the Licensed Patents and Licensed Products for research, clinical, teaching or other non-commercial purposes, and not for purposes of commercial development, use, manufacture or distribution; and
(b) subject to the confidentiality provisions set forth in Section 8, publish or otherwise disseminate any information about Licensed Patents or Licensed Know-How at any time.
2.4. Unless terminated earlier as provided in Section 13, the term of this Agreement (the "Term") shall commence on the Effective Date and shall automatically expire on the date on which the last of the Valid Claims in the Licensed Patents in the Licensed Territory expires, lapses or is declared to be invalid by a non-appealable decision of a court or other authority of competent jurisdiction through no fault or cause of Licensee.
2.5. Except as expressly provided herein, nothing in this Agreement shall be construed to grant, by implication or estoppel, any licenses under patents of Dartmouth other than the Licensed Patents. Except as expressly provided in this Agreement, under no circumstances will Licensee, as a result of this Agreement, obtain any interest in or any other right to any technology, know-how, patents, patent applications, materials or other intellectual or proprietary property of Dartmouth.
3. Sublicenses
3.1. Upon receipt of Dartmouth's prior written consent, Licensee or Affiliate of Licensee shall have the right to grant, to a third party, a Sublicense in the Field and in the Licensed Territory during the Term, but only to the extent that and during the period of time that the License is exclusive; provided that Licensee or Affiliate of Licensee shall:
(a) not receive or agree to receive anything of value in lieu of cash as consideration under such Sublicense without the prior written consent of Dartmouth;
(b) ensure that the protections and benefits provided to Dartmouth hereunder are not diminished under the Sublicense;
| DARTMOUTH | CONFIDENTIAL |
(c) prohibit any further sublicenses by the Sublicensee;
(d) obligate each Sublicensee to carry insurance and indemnify Dartmouth to the same extent as Licensee's obligations under this Agreement;
(e) make no warranties or representations on behalf of Dartmouth nor agree to any liability on behalf of Dartmouth;
(f) include in the Sublicense that, upon the expiration or termination of this Agreement for any reason, Dartmouth, at its sole discretion, may take any of the following actions: terminate the Sublicense, accept assignment of the Sublicense from Licensee, or reissue the license directly to Sublicensee from Dartmouth on reasonable terms and conditions to be negotiated in good faith; and
(g) provide Dartmouth with a true and complete copy of each sublicense agreement (and all amendments thereof) promptly after execution; provided that such agreements and amendments may be redacted to remove sensitive information of Sublicensee so long as the redacted copy contains sufficient detail for Dartmouth to ensure that the Sublicense does not materially diminish the protections and benefits afforded to Dartmouth under this Agreement, and includes the above-listed requirements.
3.2. Licensee shall remain responsible for the performance of all Sublicensees under any such Sublicense as if such performance were carried out by Licensee itself, including, without limitation, the payment of all royalties and other payments provided for hereunder, regardless of whether the terms of any Sublicense provide for such amounts to be paid by the Sublicensee to Licensee, Affiliate of Licensee, or directly to Dartmouth.
3.3. Licensee agrees that it has sole responsibility to promptly: (a) notify Dartmouth in writing of termination of any Sublicense; and (b) summarize and deliver copies of all reports provided to Licensee by Sublicensees, as redacted for any sensitive information of the Sublicensees.
4. Due Diligence
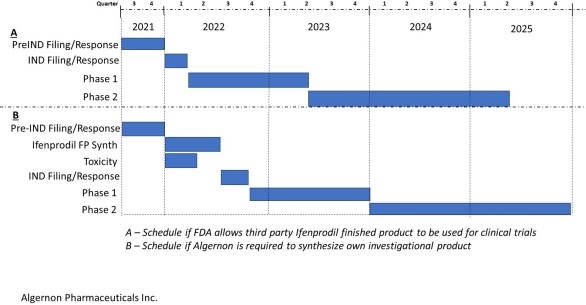
4.1. Licensee shall design a plan for developing and commercializing the Licensed Patents that includes a description of research and development, testing, government approval, manufacturing, marketing and sale or lease of Licensed Products (the "Plan"). A copy of the Plan is attached to this Agreement as Appendix B and incorporated herein by reference.
4.2. Licensee shall use Reasonable Commercial Efforts, within sixty (60) days after the Effective Date, to implement the Plan at its sole expense, and to diligently commercialize and develop markets for the Licensed Products within the Licensed Territory and in accordance with the Plan.
4.3. Within thirty (30) days after each anniversary of the Effective Date, or more often in the event of a material change to the Plan, Licensee shall provide Dartmouth with an updated and revised copy of the Plan, which shall indicate Licensee's progress to date, and a forecast and schedule of events covering the following twelve (12) months. Such updated Plan shall clearly indicate which of Licensee's products or services are Licensed Products, which Valid Claims, if any, claim such Licensed Product, and which of Licensee's products or services are Valid Claim Products.
| DARTMOUTH | CONFIDENTIAL |
4.4. Within thirty (30) days following any assignment by Licensee pursuant to Section 16.4, the assignee shall provide Dartmouth with an updated and revised copy of the Plan.
4.5. Licensee shall provide sixty (60) days advance written notice to Dartmouth in the event Licensee intends to abandon research, development and/or marketing of any Licensed Product.
4.6. It is the desire of both Dartmouth and Licensee to make Licensed Products available in the developing world, and it is the parties' common desire for Licensee to develop Licensed Products that are clinically and economically suited for use in those areas. To that end, Dartmouth and Licensee agree that Licensee shall use Reasonable Commercial Efforts to facilitate the availability of Licensed Products in low and lower-middle income countries (as defined by the World Bank) at locally affordable prices to improve access to such Licensed Products in such countries. Solely by way of example and not of limitation, Licensee may:
(a) offer Licensed Products for sale in low and lower-middle income countries at a price that is equal to Licensee's actual cost to manufacture and distribute such Licensed Products;
(b) make donations in the form of Licensed Products to governments in low income and lower-middle income countries, not-for-profit charitable organizations (such as Doctors without Borders), or other such organizations, for the purpose of treating patients in low income or lower-middle income countries; or
(c) take such other reasonable actions in support of each party's goal of making Licensed Products available in the developing world.
4.7. Unless otherwise agreed to in writing by the parties, Dartmouth shall be entitled to terminate this Agreement upon the occurrence of any of the following:
(a) Licensee fails to provide the written reports as provided in Sections 4.1 or 4.3, and does not cure such failure within sixty (60) days after Licensee's receipt of written notice thereof from Dartmouth;
(b) Licensee gives notice pursuant to Section 4.5; or
(c) Licensee or Affiliate of Licensee has failed to:
(i) file an IND for a Licensed Product, or has failed to cause an IND to be filed for a Licensed Product, within two (2) years after the Effective Date; or
| DARTMOUTH | CONFIDENTIAL |
(ii) following the filing of an IND for a Licensed Product, demonstrate ongoing clinical development of such Licensed Product, which shall be evidenced by the failure to conduct at least one of the following activities in any given twelve (12) month period starting from the date of such IND filing and ending upon the First Sale in the United States:
(A) having manufactured a Licensed Product suitable for clinical trials under an approved IND;
(B) having actively engaged in study preparation, implementation, results analysis or reporting of a Phase I Clinical Trial, Phase I/II Clinical Trial, Phase II Clinical Trial, Phase III Clinical Trial, or Pivotal Clinical Trial with respect to a Licensed Product underway;
(C) having filed an NDA or BLA for a Licensed Product in the United States;
(D) following NDA or BLA approval of a Licensed Product, having launched or sold a Licensed Product in the United States.
5. Consideration.
5.1. License Issuance Fee. Licensee shall pay to Dartmouth within ten (10) days after the Effective Date a one-time lump sum non-refundable license issue fee of [****].
5.2. License Maintenance Fee. Licensee shall pay to Dartmouth an annual license maintenance fee (the "LMF") of [****], which shall be due on the first anniversary of the Effective Date and every anniversary of the Effective Date thereafter. Licensee shall continue to pay the LMF until the end of the Term.
5.3. Milestone Fees. Licensee shall pay to Dartmouth the following milestone fee for each Licensed Product developed by Licensee, a Sublicensee, or an Affiliate of Licensee:
(a) a non-refundable milestone fee of [****] upon Licensee's receipt of NDA or BLA approval in the United States.
5.4. Earned Royalty. As partial consideration for the License, Licensee shall pay to Dartmouth an earned royalty on worldwide cumulative Net Sales of Licensed Products by Licensee, the Sublicensees or Affiliates of Licensee ("Earned Royalty") of [****].
None of the License Issue Fee set forth in Section 5.1, the LMF set forth in Section 5.2, nor the milestone fees set forth in Section 5.3 shall be credited against Earned Royalties payable under Section 5.4.
| DARTMOUTH | CONFIDENTIAL |
5.5. Minimum Royalty Payment. Licensee agrees to pay to Dartmouth an annual Minimum Royalty Payment ("MRP") of [****], payable for each calendar year commencing on the first January 1 to occur after the date of the First Sale and continuing on each January 1 thereafter. Licensee shall continue to pay the MRP until the end of the Term. With respect to each calendar year in which a MRP is payable hereunder, Licensee shall pay to Dartmouth twenty-five percent (25%) of such MRP within thirty (30) days after the end of each calendar quarter in such calendar year. Dartmouth shall fully credit each MRP made against any Earned Royalties due and payable by Licensee in the same calendar year.
5.6. Sublicense Income Fee. Licensee shall pay to Dartmouth a percentage of any Sublicense Income received by Licensee, as and when received by Licensee, as follows:
| Event | Percentage of Sublicense Income |
| Sublicense Income received by Company prior to initiation of a Phase I clinical trial or Phase I/II clinical trial by Company, sublicensees or Company's Affiliates of a Licensed Product | [****] |
| Sublicense Income received by Company after the initiation of a Phase I clinical trial or Phase I/II clinical trial for a Licensed Product but prior to initiation of the first Phase III clinical trial or Pivotal Clinical Trial by Company, sublicensee or Company's Affiliates of a Licensed Product | [****] |
| Sublicense Income received by Licensee after initiation of the first Phase III clinical trial or pivotal clinical trial by licensee, sublicensee or Company's Affiliates of a Licensed Product | [****] |
5.7. Timing of Payments. Licensee shall pay, or cause to be paid, all Earned Royalties accruing to Dartmouth within thirty (30) days from the end of each calendar quarter (March 31, June 30, September 30 and December 31), beginning in the first calendar quarter in which Net Sales occur. Licensee shall report all Earned Royalties and other payments accruing to Dartmouth on a quarterly basis, but shall defer payments accruing to Dartmouth that do not, in total, exceed [****] in any given quarter until the earlier of (a) the end of the calendar year, or (b) the quarter upon which the cumulative accrued Earned Royalties or other payments exceed [****].
| DARTMOUTH | CONFIDENTIAL |
5.8. Payment Currency; Interest; Collection. All payments due under this Agreement shall be paid by Licensee to Dartmouth in United States Dollars. In the event that conversion from foreign currency is required in calculating a payment under this Agreement, the exchange rate used shall be the Interbank rate quoted by Citibank at the time the payment is due. If overdue, all payments due to Dartmouth under this Agreement shall bear interest until payment at a per annum rate two hundred basis points above the prime rate in effect at Citibank on the due date and Dartmouth shall be entitled to recover from Licensee reasonable attorneys' fees and costs related to the collection of any payments due Dartmouth hereunder following Licensee's failure to pay. The payment of such interest shall not foreclose Dartmouth from exercising any other right it may have as a consequence of the failure of Licensee to make any payment when due. If Licensee is required by law to withhold any tax from the payment of royalties to Dartmouth, Licensee will, at Dartmouth's request, provide documentation showing that the amount withheld was paid to the appropriate tax authorities.
6. Patent Costs.
6.1. Upon execution of this Agreement, Licensee shall reimburse Dartmouth for all patent
costs paid by or invoiced to Dartmouth on or before the Effective Date, which amount as of the Effective Date is [****].
6.2. Licensee shall reimburse Dartmouth for all patent costs incurred by Dartmouth after the Effective Date within thirty (30) days following the date an invoice is sent from Dartmouth to Licensee in respect of such patent costs. At Dartmouth's request, Licensee will receive invoices directly from Dartmouth's patent counsel for all patent costs invoiced after the Effective Date and will timely pay all such invoices. Dartmouth will receive copies of all invoices. Failure to timely pay patent costs is a breach of this Agreement.
6.3 The costs described in Sections 6.1 and 6.2 shall include, but are not limited to, any past, present and future taxes, annuities, working fees, maintenance fees, renewal and extension charges.
7. Patent Challenges
7.1. In the event that Licensee or any of its Affiliates or Sublicensees brings a Patent Challenge in the Licensed Territory, or Licensee or any of its Affiliates or Sublicensees assists another party in bringing a Patent Challenge in the Licensed Territory (except as required under a court order or subpoena), then the following provisions shall apply:
(a) All payments due to Dartmouth under this Agreement, other than patent costs, shall be tripled during the pendency of such Patent Challenge and shall remain payable to Dartmouth when due; provided that, notwithstanding the foregoing, if a First Sale has been made in the Licensed Territory, then only Earned Royalties due in the Licensed Territory shall be tripled during the pendency of such Patent Challenge.
| DARTMOUTH | CONFIDENTIAL |
(b) If such Patent Challenge is inconclusive or results in a determination that at least one challenged claim is both valid and infringed, (i) all payments due to Dartmouth under this Agreement, other than patent costs, shall be tripled for the remainder of the Term; provided that, notwithstanding the foregoing, once a First Sale has been made in the Licensed Territory, then only Earned Royalties due in the Licensed Territory shall be tripled, and (ii) Licensee shall promptly reimburse Dartmouth for all legal fees and expenses incurred in Dartmouth's defense against such Patent Challenge.
(c) In the event that such Patent Challenge is successful, Licensee will have no right to recoup any payments made prior to the final, non-appealable determination of a court of competent jurisdiction.
7.2. Neither Licensee nor any of its Affiliates or Sublicensees shall bring a Patent Challenge without first providing Dartmouth at least thirty (30) days prior written notice setting forth (a) precisely which claims and patents are being challenged or claimed not to be infringed, (b) a clear statement of the factual and legal basis for the challenge, and (c) an identification of all prior art and other matter believed to invalidate any claim of the Licensed Patent or which supports the claim that the Licensed Patent is not infringed.
8. Confidentiality and Publicity
8.1. Subject to the parties' rights and obligations pursuant to this Agreement, Dartmouth and Licensee agree that during the Term and for five (5) years thereafter, the recipient of the other party's Confidential Information:
(a) will keep confidential and will cause its Affiliates, Sublicensees and its and their respective employees, agents, contractors and associate (collectively, "Representatives") that have access to the disclosing party's Confidential Information to keep confidential such Confidential Information by taking whatever action the recipient would take to preserve the confidentiality of its own Confidential Information, which in no event shall be less than reasonable care; and
(b) may disclose the disclosing party's Confidential Information to its Representatives that need such Confidential Information to fulfill the obligations set forth in this Agreement; provided that all such Representatives shall be bound by confidentiality restrictions at least as stringent as those set forth herein with respect to the disclosing party's Confidential Information; and provided further that recipient shall be responsible for the breach of the confidentiality obligations hereunder by its Representatives; and
(c) will not use the other party's Confidential Information other than as expressly permitted or contemplated by this Agreement or disclose the other party's Confidential Information to any third parties except as expressly permitted or contemplated by this Agreement without advance written permission from the other party; and
| DARTMOUTH | CONFIDENTIAL |
(d) will, within thirty (30) days of termination of this Agreement, return all the Confidential Information disclosed to it by the other party pursuant to this Agreement except for one copy which may be retained by the recipient for monitoring compliance with this Section 8.1 and any surviving clauses.
8.2. The obligations of confidentiality described above shall not pertain to that part of the Confidential Information that:
(a) is shown to have been known to without restriction or developed by the recipient prior to the disclosure by the disclosing party as evidenced by recipient's competent written records; or
(b) is at the time of disclosure or has become thereafter publicly known through no fault or omission attributable to the recipient; or
(c) is rightfully given to the recipient from sources independent of the disclosing party without restriction; or
(d) is independently developed by recipient without use of or access to the Confidential Information of the disclosing party as shown by the competent written records of recipient.
8.3. In the event Confidential Information is required to be disclosed by law, regulation, court order or other legal requirement that purports to compel disclosure of any Confidential Information, recipient will promptly notify the disclosing party upon learning of any such legal requirement, and cooperate with the disclosing party in the exercise of its right to protect the confidentiality of its Confidential Information before any tribunal or governmental agency. If recipient is required by law to disclose Confidential Information, recipient shall (a) disclose only such Confidential Information as is specifically required,
(b) disclose only to such persons as specifically required, and (c) use reasonable efforts to obtain confidential treatment for any Confidential Information that is disclosed.
8.4. The financial terms of this Agreement constitute Confidential Information of each of the parties.
8.5. Notwithstanding any other provision set forth herein, Licensee shall be permitted to disclose Dartmouth's Confidential Information and this Agreement to any potential financing source, acquirer, sublicensee or strategic partner as long as such person or entity has executed a confidentiality agreement with Licensee that contains confidentiality provisions at least as stringent as those contained herein with respect to Dartmouth's Confidential Information.
9. Reports, Records and Inspections; Compliance with Law
9.1. Reports. Licensee shall (i) within thirty (30) days after the end of each calendar year; and
(ii) from and after Net Sales first occur, within thirty (30) days after each calendar quarter, provide Dartmouth with a written report detailing the Net Sales made by Licensee, its Sublicensees and Affiliates of Licensee on Licensed Products during the preceding calendar quarter and calculating the payments due pursuant to Section 5. Net Sales of Licensed Products shall be deemed to have occurred when Licensee receives the Net Sales for such Licensed Products. Each such report shall be signed by an officer of Licensee (or the officer's designee), and must include:
| DARTMOUTH | CONFIDENTIAL |
(a) the number or amount, as appropriate, of Licensed Products manufactured, sold or otherwise transferred by Licensee, Sublicensees and Affiliates of Licensee;
(b) a calculation of Net Sales for the applicable reporting period in each country, including the gross invoice prices charged for the Licensed Products and any permitted deductions made pursuant to Section 1.19;
(c) a calculation of all royalties or other payments due hereunder, including any exchange rates used for conversion; and
(d) names and addresses of all Sublicensees and the type and amount of any Sublicense Income received from each Sublicensee;
(e) identification of any milestone event that occurred since the date of the last report and the date of such occurrence; and
(f) identification of any changes in Inventor Agreement that went into effect during the previous reporting period.
9.2. Records. Licensee, Sublicensees, and Affiliates of Licensee shall keep and maintain complete and accurate records and books containing an accurate accounting of all data in sufficient detail to enable verification of all royalties and other payments due Dartmouth under this Agreement. Licensee shall preserve such books and records for three (3) years after the calendar year to which they pertain. Such books and records shall be open to inspection by Dartmouth or an independent certified public accountant selected by Dartmouth, at Dartmouth's expense, during normal business hours upon ten (10) business days' prior written notice, for the purpose of verifying the accuracy of the reports and computations rendered by Licensee. In the event Licensee underpaid the amounts due to Dartmouth with respect to the audited period by more than five percent (5%), Licensee shall pay the reasonable cost of such examination, together with the deficiency not previously paid and interest from the due date of such payment, calculated at the rate set forth in Section 5.7, within thirty (30) days of receiving notice thereof from Dartmouth.
9.3. Financial Statements. On or before the one hundred and twentieth (120th) day following the close of Licensee's fiscal year, Licensee shall provide Dartmouth with Licensee's financial statements for the preceding fiscal year including, at a minimum, a balance sheet and an income statement and, if Licensee's financial statements for such year have been audited, Licensee shall provide such audited financial statements to Dartmouth.
9.4. Export Compliance. Licensee represents and warrants that it will comply, and will cause its Sublicensees and Affiliates to comply with all applicable local, state, federal and international laws and regulations relating to the development, manufacture, use, sale and importation of Licensed Products. Without limiting the foregoing, Licensee represents and warrants on behalf of itself and its Sublicensees and Affiliates, that they shall comply with all United States laws and regulations controlling the export of certain commodities and technical data, including without limitation all Export Administration Regulations of the United States Department of Commerce, and all other export control laws and regulations. Licensee agrees to be responsible for any violation of such laws and regulations by itself or its Sublicensees or Affiliates, and further agrees to indemnify, defend and hold the Indemnitees (as defined below) harmless, in accordance with Sections 14.4 and 14.5, for the consequences of any such violation.
| DARTMOUTH | CONFIDENTIAL |
10. Patent Protection
10.1. Any and all United States patent applications, and resulting issued patents contained in the Licensed Patents, shall remain the property of Dartmouth.
10.2. All patent applications under the Licensed Patents shall be prepared, prosecuted, filed and maintained by independent patent counsel chosen by Dartmouth and reasonably acceptable to Licensee. The independent patent counsel shall be ultimately responsible to Dartmouth. Dartmouth shall instruct patent counsel to keep both Dartmouth and Licensee fully informed of the progress of all patent applications and patents, and to give both Dartmouth and Licensee reasonable opportunity to comment on the type and scope of useful claims and the nature of supporting disclosures. Dartmouth will not finally abandon any patent application for which Licensee is bearing expenses without Licensee's prior written consent. Dartmouth shall have no liability to Licensee for damages, whether direct, indirect, incidental, consequential or otherwise, allegedly arising from its good faith decisions, actions and omissions in connection with such prosecution.
10.3. Licensee shall mark, and shall require Affiliates of Licensee and Sublicensees to mark, all Valid Claim Products that are tangible products with the numbers of all Licensed Patents that cover the Valid Claim Products. Without limiting the foregoing, all Valid Claim Products shall be marked in such a manner as to conform with the patent marking notices required by the laws, regulations or guidelines effective in any country where such Valid Claim Products are made, sold, used or shipped.
11. Infringement and Litigation
11.1. Each party shall promptly notify the other party in writing in the event that it obtains knowledge of infringing activity by third parties, or is sued or threatened with an infringement suit, in any country in the Licensed Territory as a result of activities that concern the Licensed Patents, and shall supply the other party with documentation of the infringing activities that it possesses.
11.2. During the Term:
(a) Licensee shall have the first right to defend the Licensed Patents against infringement or challenges in the Field and in the Licensed Territory by third parties, which includes bringing any legal action for infringement and defending any counter claim of invalidity or action of a third party for declaratory judgment for non-infringement or non-challenge. If, in the reasonable opinion of both Licensee's and Dartmouth's respective counsel, Dartmouth is required to be a named party to any such suit for standing purposes, Licensee may join Dartmouth as a party; provided, however, that (i) Dartmouth shall not be the first named party in any such action, (ii) the pleadings and any public statements about the action shall state that the action is being pursued by Licensee and that Licensee has joined Dartmouth as a party, and (iii) Licensee shall keep Dartmouth reasonably apprised of all developments in any such action. Licensee may settle such suits solely in its own name and solely at its own expense and through counsel of its own selection; provided, however, that no settlement shall be entered without Dartmouth's prior written consent, such consent not to be unreasonably withheld, conditioned or delayed. Without limiting the foregoing, Dartmouth may withhold its consent to any settlement that would in any manner constitute or incorporate an admission of liability by Dartmouth or require Dartmouth to take or refrain from taking any action. Licensee shall bear the expense of such legal actions. Except for providing reasonable assistance, at the request and expense of Licensee, Dartmouth shall have no obligation regarding the legal actions described in Section 11.2 unless required to participate by law. However, Dartmouth shall have the right to participate in any such action through its own counsel and at its own expense. Any recovery shall first be applied to Licensee's out of pocket expenses and second shall be applied to Dartmouth's out of pocket expenses, including legal fees. With respect to any excess recovery received by Licensee after the payment of such out-of-pocket expenses that constitute damages for lost sales of Licensed Products, such recovery shall be treated as Net Sales for which Dartmouth shall be paid Earned Royalties. Any excess recovery received by Licensee after the payment of such out-of-pocket expenses that does not constitute damages for lost sales of Licensed Products (including treble or punitive damages) shall be treated as Sublicense Income.
| DARTMOUTH | CONFIDENTIAL |
(b) In the event Licensee fails to initiate and pursue or participate in the actions described in Section 11.2(a) in a particular country within sixty (60) days after (a) notification of infringement from Dartmouth, or (b) the date Licensee otherwise first becomes aware of an infringement, whichever is earlier, Dartmouth may, in its sole discretion, convert the License granted in Section 2 solely with respect to such particular country to a nonexclusive license, and issue licenses to third parties under the Licensed Patents to make, have made, use, sell, have sold, import, export, or practice Licensed Products within the Field in such particular country. Additionally, Dartmouth shall have the right to initiate such legal action at its own expense and Dartmouth may use the name of Licensee as party plaintiff to uphold the Licensed Patents. In such case, Licensee shall provide reasonable assistance to Dartmouth if requested to do so. Dartmouth may settle such actions solely through its own counsel. Any recovery shall be retained by Dartmouth. This Section 11.2(b) shall be of no force or effect during any such period in which a valid Sublicense with respect to the Licensed Patents is in effect; provided that Licensee hereby agrees during any such period to use commercially reasonable efforts, itself or through its Sublicensees, to enforce the Licensed Patents against infringers, and in the event that neither Licensee nor a relevant Sublicensee intends to take action to terminate an ongoing infringement after written request of Dartmouth to do so, Licensee and/or its relevant Sublicensee shall discuss in good faith with Dartmouth actions to be taken to address Dartmouth's reasonable concerns.
| DARTMOUTH | CONFIDENTIAL |
(c) In the event Licensee is permanently enjoined from exercising its License under this Agreement pursuant to an infringement action brought by a third party, or if both Licensee and Dartmouth elect not to undertake the defense or settlement of a suit alleging infringement for a period of six (6) months from notice of such suit, then Dartmouth shall have the right to remove the applicable Licensed Patent in the country where the suit was filed from the scope of this Agreement following thirty
(30) days' written notice to Licensee. This Section 11.2(c) shall be of no force or effect during any such period in which a valid Sublicense with respect to the Licensed Patents is in effect.
(d) Notwithstanding the foregoing, neither Licensee nor Dartmouth shall take any action to enforce the Licensed Patents in low income and lower-middle income countries (as designated by the World Bank (www.worldbank.org)) where such action is intended to prevent the sale of Licensed Products in any such countries. However, Licensee and/or Dartmouth may take such action in any such country, provided that such action is intended to prevent the manufacturing of Licensed Products for export to countries that are not low income countries.
12. Use of Dartmouth's Name
12.1. Licensee shall not use the name "Dartmouth" or "Dartmouth College," nor any variation or adaptation thereof, nor any trademark, tradename or other designation owned by Dartmouth, nor the names of any of its trustees, officers, faculty, students, employees or agents, for any purpose without the prior written consent of Dartmouth in each instance, such consent to be granted or withheld by Dartmouth in its sole discretion, except that Licensee may state that it has licensed from Dartmouth one or more of the patents and/or applications comprising the Licensed Patents.
13. Termination
13.1. Dartmouth shall have the right to terminate this Agreement upon written notice to Licensee as provided in Section 4.7, or in the event Licensee:
(a) fails to make any payment whatsoever due and payable pursuant to this Agreement unless Licensee shall make all such payments within the ten (10) day period after receipt of written notice to do so from Dartmouth;
(b) breaches its other obligations under this Agreement, and such breach is not cured within the thirty (30) day period after receipt of written notice of such breach from Dartmouth; or
(c) fails to obtain or maintain adequate insurance as described in Section 14.6 within the thirty (30) day period after receipt of written notice from Dartmouth.
| DARTMOUTH | CONFIDENTIAL |
13.2. This Agreement shall terminate automatically without any notice to Licensee in the event Licensee becomes Insolvent.
13.3. Licensee shall have the right to terminate this Agreement upon written notice to Dartmouth:
(a) at any time on six (6) months' notice to Dartmouth, provided that payment of all amounts due to Dartmouth through the effective date of termination has been made;
(b) in the event that Dartmouth breaches any of its obligations in Sections 2.1, 2.5 or 8 and such breach is not cured within the thirty (30) day period after receipt of written notice of such breach from Licensee.
13.4. Upon termination of this Agreement, for any reason, all rights and licenses granted to Licensee under the terms of this Agreement are terminated. Upon such termination, Licensee shall cease to make, have made, use, develop, sell, have sold, perform, practice, import and export Licensed Products. Within forty-five (45) days of the effective date of termination, Licensee shall return to Dartmouth:
(a) all materials relating to or containing the Licensed Patents or Confidential Information disclosed by Dartmouth;
(b) the last report required under Section 3 or Section 9; and
(c) all payments incurred up to the effective date of termination.
13.5. Termination of this Agreement shall not affect the rights or obligations accrued prior to the effective date of such termination and specifically Licensee's obligation to pay all royalties and other payments specified by Section 5 that have accrued prior to the effective date of such termination. Termination of this Agreement shall not terminate or otherwise affect any Sublicense previously provided or granted by Licensee, provided that Dartmouth's obligations and responsibilities under such Sublicenses would not be materially different than its obligations and responsibilities under this Agreement, and further provided that Dartmouth is entitled to receive the payments otherwise payable to Licensee under such Sublicenses. The following provisions shall survive any termination: Sections 1, 2.5, 5.7, 5.8, 8, 9.2 - 9.4, 12, 13.4 - 13.8, and 14 - 16. Section 14.6 shall survive for ten (10) years past the last sale of any Licensed Product. The parties agree that claims giving rise to indemnification may arise after the Term or termination of the License granted herein.
13.6. The rights provided in this Section 13 shall be in addition and without prejudice to any other rights, whether at law or in equity, which the parties may have with respect to any default or breach of the provisions of this Agreement.
13.7. Waiver by either party of one or more defaults or breaches shall not deprive such party of its right with respect to any subsequent default or breach.
13.8. Upon termination of this Agreement for any reason other than breach by Dartmouth, Licensee shall permit Dartmouth and its future licensees to utilize, reference and otherwise have the benefit of all regulatory approvals of, or clinical trials or other studies conducted on, and all filings made with regulatory agencies with respect to, the Licensed Products. In addition, at Dartmouth's request, Licensee shall deliver to Dartmouth within three (3) months of such request all records required by regulatory authorities to be maintained with respect to the sale, storage, handling, shipping and use of the Licensed Products, all reimbursement approval files, all documents, data and information related to clinical trials and other studies of Licensed Products, any other data, techniques, know-how and other information developed or generated that relate to the Licensed Patents, all copies and facsimiles of such materials, documents, information and files and all inventories of Licensed Product, whether commercial, clinical or research products. Dartmouth agrees that, subject to the provisions of Section 8, Licensee may retain one copy thereof to the extent Licensee is required by law to maintain such copy. Dartmouth and Licensee hereby agree that this Section 13.8 shall not be deemed to require the transfer or provision to Dartmouth of any information, documentation or filings generated by any Sublicensee, or on behalf of any Sublicensee by any party other than Licensee, in either case in the course of performance of its Sublicense, or of any rights in and to the foregoing.
| DARTMOUTH | CONFIDENTIAL |
14. Limitation of Liability; Indemnification; Insurance
14.1. No Warranty. THE LICENSES GRANTED HEREIN ARE PROVIDED "AS IS" AND WITHOUT WARRANTY OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, NON-INFRINGEMENT, VALIDITY OF ANY OF THE DARTMOUTH PATENT CLAIMS, WHETHER ISSUED OR PENDING, AND THE ABSENCE OF LATENT OR OTHER DEFECTS, WHETHER OR NOT DISCOVERABLE, OR ANY OTHER WARRANTY, EXPRESS OR IMPLIED. DARTMOUTH MAKES NO REPRESENTATION OR WARRANTY THAT THE EXERCISE OF ANY RIGHTS TO ANY LICENSED PRODUCT, LICENSED KNOW-HOW, OR LICENSED PATENT WILL NOT INFRINGE ANY PATENT OR OTHER PROPRIETARY RIGHTS OF DARTMOUTH OR OF ANY THIRD PARTY.
14.2. Limitation of Liability. IN NO EVENT SHALL DARTMOUTH OR ANY OF ITS AFFILIATES OR ANY OF THEIR RESPECTIVE TRUSTEES, DIRECTORS, OFFICERS, EMPLOYEES, STUDENTS AND AGENTS BE LIABLE TO LICENSEE, ANY SUBLICENSEE AND ANY AFFILIATE OF LICENSEE FOR ANY INCIDENTAL, SPECIAL OR CONSEQUENTIAL DAMAGES OF ANY KIND ARISING IN ANY WAY OUT OF OR IN CONNECTION WITH THIS AGREEMENT OR THE RIGHTS GRANTED HEREUNDER, HOWEVER CAUSED AND ON ANY THEORY OF LIABILITY, INCLUDING WITHOUT LIMITATION ECONOMIC DAMAGES OR INJURY TO PROPERTY OR LOST PROFITS, REGARDLESS OF WHETHER DARTMOUTH SHALL BE ADVISED, SHALL HAVE OTHER REASON TO KNOW, OR IN FACT SHALL KNOW OF THE POSSIBILITY OF THE FOREGOING.
14.3. Nothing in this Agreement shall be construed as:
(a) a warranty or representation by Dartmouth as to the validity or scope of any Licensed Patent or Licensed Know-How;
| DARTMOUTH | CONFIDENTIAL |
(b) a warranty or representation that anything made, used, sold or otherwise disposed of under any license granted in this Agreement is or shall be free from infringement of patents or of any intellectual property of Dartmouth or of any third parties;
(c) an obligation to bring or prosecute actions or suits against third parties for patent infringement or infringement of any other intellectual property rights; or
(d) conferring by implication, estoppel or otherwise any license or rights under any patents or any other intellectual property of Dartmouth other than Licensed Patents or Licensed Know-How.
14.4. Indemnification. Licensee, Sublicensees and Affiliates of Licensee shall indemnify, hold harmless and defend Dartmouth, its trustees, officers, employees, students, and agents, and Inventor and their employers (collectively, the "Indemnitees") against any liability, damage, judgement, loss or expense (including reasonable attorney's fees and expenses of litigation) incurred by or imposed upon the Indemnitees or any one of them in connection with any claims, suits, actions, or demands brought by a third party under any theory of liability (including without limitation, actions in the form of tort, warranty, or strict liability, or violation of any law, and regardless of whether such action has any factual basis) arising out of or relating to (a) any failure by Licensee, Sublicensee, and or Affiliate of Licensee to perform any of their obligations under this Agreement and/or under any Sublicense, or (b) any defect in any Licensed Product sold by Licensee, Affiliate of Licensee, or Sublicensee, or (c) the gross negligence, willful misconduct or fraud of Licensee. Licensee agrees that neither Dartmouth nor any Indemnitee shall have any liability to Licensee or any person asserting claims on behalf of or in right of Licensee in connection with or as a result of the License, except to the extent arising from the gross negligence, willful misconduct or fraud of Dartmouth.
14.5. Indemnification Procedure. Licensee agrees, at its own expense, to provide attorneys reasonably acceptable to Dartmouth to defend against any actions brought or filed against any Indemnitee, whether or not such actions are rightfully brought; provided, however, that any Indemnitee shall have the right to retain its own counsel, at its own expense, if representation of such Indemnitee by counsel retained by Licensee would be inappropriate because of actual or potential conflicts of interests of such Indemnitee and any other party represented by such counsel. Dartmouth will use reasonable efforts to notify Licensee in writing within thirty (30) days of the assertion by a third party of any claim that is subject to indemnification under this Section 14.5. Failure to notify Licensee will not waive any of Licensee's indemnity obligations with respect to such claim except to the extent such failure materially prejudices the ability of Licensee to defend such claim. The parties will cooperate with each other in the defense and settlement of any such claim, at Licensee's sole expense. Licensee agrees to keep Dartmouth informed of the progress in the defense and disposition of such indemnified claim and to consult with Dartmouth prior to any proposed settlement. In the event Licensee does not accept the defense of any matter, Dartmouth will have the right to defend such matter and all of Dartmouth's reasonable costs, expenses, liabilities and damages (including any amounts payable in settlement) will be promptly reimbursed by Licensee. Licensee will not settle any matter under this Section 14.5 without Dartmouth's prior written consent, which consent will not be unreasonably withheld, conditioned or delayed.
| DARTMOUTH | CONFIDENTIAL |
14.6. Insurance. Licensee, at its sole cost and expense, shall insure its activities under this Agreement and obtain, keep in force and maintain insurance or an equivalent program of self-insurance as follows:
(a) comprehensive or commercial general liability insurance (contractual liability included) with limits of at least:
(i) each occurrence - One Million Dollars (US $1,000,000);
(ii) products/completed operations aggregate - Five Million Dollars (US $5,000,000);
(iii) personal and advertising injury - One Million Dollars (US $1,000,000); and
(iv) general aggregate (commercial form only) - Five Million Dollars (US $5,000,000).
(b) cyber liability insurance with limits of at least Five Million Dollars ($5,000,000) per occurrence and Ten Million Dollars ($10,000,000) in the aggregate.
The coverage and limits referred to above will not in any way limit the liability of Licensee, and all rights of subrogation will be waived against Dartmouth and its insurers.
(c) Licensee shall, within ninety (90) days of the Effective Date, furnish Dartmouth with certificates of insurance showing compliance with all requirements. The policy or policies of insurance specified herein shall be issued by an insurance carrier with an A.M. Best rating of "A" (or an comparable rating in the jurisdiction in which the insurance carrier operates) or better and shall name Dartmouth as an additional insured with respect to Licensee's performance of this Agreement. Such certificates shall: (i) provide for thirty (30) day advance written notice to Dartmouth of any modification; and (ii) include a provision that the coverage shall be primary and shall not participate with nor shall be excess over any valid and collectable insurance or program of self-insurance carried or maintained by Dartmouth.
(d) Dartmouth reserves the right to require additional policies of insurance and/or increased coverage limits where appropriate and reasonable in light of Licensee's business operations and availability of coverage.
Notwithstanding the foregoing provisions in this Section, Licensee shall only be required to obtain, keep in force and maintain insurance if the Licensee receives NDA or BLA approval in the United States and is selling, marketing, or distributing Licensed Product in the Licensed Territory.
15. Inventor Agreements
15.1. If Licensee and Inventor enter into an Inventor Agreement, Licensee shall so notify Dartmouth in writing within thirty (30) days after the execution of such Inventor Agreement. The Licensee acknowledges that: (i) Inventor is a faculty member of Dartmouth; (ii) Inventor is subject to certain policies of Dartmouth, including policies concerning consulting, conflicts of interest, and intellectual property ("Dartmouth Policies"); and (iii) to the extent any provision of the Inventor Agreement conflicts with Dartmouth Policies, or imposes obligations or responsibilities compliance with which would require Inventor to act in violation of Dartmouth Policies, the provisions of the Dartmouth Policies shall prevail.
| DARTMOUTH | CONFIDENTIAL |
16. Miscellaneous
16.1. Governing Law; Venue and Consent to Jurisdiction. This Agreement, including the appendices and all other attachments, and all disputes arising in connection with this Agreement and the subject matter herein, will be governed by and construed in accordance with the laws of the State of New Hampshire, without giving effect to its conflict of laws principles, except that questions affecting the construction and effect of any patent shall be determined by the law of the country in which the patent shall have been granted.
16.2. Attorneys' Fees. In the event of litigation between the parties arising from or related to this Agreement, the prevailing party shall be entitled to recovery of all reasonable costs and attorneys' fees.
16.3. Dispute Resolution. If any dispute or disagreement arises between the parties, then, prior to initiating any litigation or formal dispute resolution, the parties will follow the following procedures in an attempt to resolve the dispute or disagreement:
(a) The party claiming that such a dispute exists will give notice in writing ("Notice of Dispute") to the other party of the nature of the dispute.
(b) Within fourteen (14) days after receipt of a Notice of Dispute, the Director of the Dartmouth Technology Transfer Office and the Chief Executive Officer of Licensee (or a person that the Chief Executive Officer of Licensee designates) will meet in person or by teleconference and exchange written summaries reflecting, in reasonable detail, the nature and extent of the dispute, and at this meeting they will use their reasonable endeavors to resolve the dispute.
(c) If the persons referred to in Section 16.3(b) are unable to resolve the dispute during the meeting described in Section 16.3(b) or if for any reason such meeting does not take place within the period specified in Section 16.3(b), then the dispute will be referred to the Office of the General Counsel of Dartmouth, and the Chief Executive Officer of Licensee (or a person that the Chief Executive Officer of Licensee designates), who will meet at a mutually agreed-upon time and location for the purpose of resolving such dispute.
(d) If, within a further period of thirty (30) days, or if in any event within ninety (90) days of initial receipt of the Notice of Dispute, the dispute has not been resolved, or if, for any reason, the meeting described in Section 16.3(c) has not been held within ninety (90) days of initial receipt of the Notice of Dispute, then either party may refer such dispute to arbitration in accordance with this Section 16.3(d). All arbitration proceedings shall be conducted in accordance with the Comprehensive Arbitration Rules and Procedures of JAMS before a single arbitrator selected by the parties, or if the parties do not agree upon the selection an arbitrator within thirty (30) days following the date on which the non-referring party has been notified of the arbitration, a single arbitrator chosen in accordance with such rules. The place of arbitration shall be Concord, New Hampshire. The arbitration shall be conducted in English. The administrative fees of the arbitration and the arbitrator's fees shall be shared equally by the parties, provided that the arbitrator may award costs, as well as attorneys' fees, to the prevailing party in addition to any award of damages or other relief. The arbitrator shall be bound by any pertinent provisions contained in this Agreement. The arbitrator's decision shall be final and binding on both parties and either party may apply to any court having jurisdiction for enforcement of the decision. The parties shall keep the claim, evidence, proceedings and decision in the arbitration confidential except as to their professional advisors, as required to enforce or appeal the decision, or as otherwise required by law or regulation.
| DARTMOUTH | CONFIDENTIAL |
(e) Nothing in this Section 16.3 shall limit the right of a party to seek injunctive or other equitable relief from a court having jurisdiction with respect to any actual or threatened breach of this Agreement.
16.4. Assignment. This Agreement may not be amended or modified except by written agreement executed by each of the parties. This Agreement is personal to Licensee and shall not be assigned by Licensee without the prior written consent of Dartmouth; provided that Licensee may assign this Agreement without the consent of Dartmouth to an Affiliate or to a purchaser of all, or substantially all, of the assets or equity securities of Licensee (whether through merger, consolidation, assignment or otherwise); provided that Licensee must give Dartmouth thirty (30) days prior written notice of any such assignment. Dartmouth shall not sell, assign or otherwise transfer any of its rights to any of the Licensed Patents unless either (a) the purchaser or assignee of such rights has acknowledged and agreed with Licensee in writing that such purchaser or assignee will assume and perform all of Dartmouth's obligations under this Agreement and that such sale, assignment and transfer will not adversely affect any of Licensee's rights under this Agreement, or (b) Licensee has provided its prior written consent to such sale, assignment or transfer. Any attempted assignment in contravention of this Section 16.4 shall be null and void.
16.5. Notices. All notices or other communications required or permitted to be given hereunder shall be in writing and shall be deemed to have been delivered to a party upon: (a) personal delivery to that party; (b) if simultaneously mailed as provided herein, upon: (i) electronically confirmed delivery by facsimile to the telephone number provided by the party for such purposes; or (ii) electronic mail transmission to the electronic mailbox provided by the party for such purposes; (c) upon deposit for overnight delivery with a bonded courier holding itself out to the public as providing such services, with charges prepaid; or (d) four (4) business days following deposit of certified or registered mail in Canada or the United States, postage prepaid, and in any case addressed to the party's address set forth below, or to any other address that the party provides by notice, in accordance with this Section to the other party:
| DARTMOUTH | CONFIDENTIAL |
If to Dartmouth:
ATTN: [****]
Phone: [****] Fax: [****] Email: [****]
If to Licensee:
ATTN: [****]
Phone: [****]
E-mail: [****]
16.6. Entire Agreement; Order of Precedence. This Agreement, including all appendices hereto, constitutes the entire agreement of the parties, and supersedes any prior and contemporaneous agreements, either oral or written, between the parties hereto with respect to the subject matter hereof. Each party acknowledges that no representations, inducements, promises, or agreements, oral or otherwise, have been made by the other party, or anyone acting on behalf of the other party, that are not embodied herein, and that no other agreement, statement, or promise not contained in this Agreement shall be valid or binding. The terms of this Agreement take precedence over the terms of any purchase order or similar document issued by a party, which may be accepted by the other Party for administrative convenience only.
16.7. Language. The parties agree that they will contract in the English language and that there shall be no requirement to translate this Agreement or any of the documents incorporated into this Agreement into any other language. In the event of any inconsistencies between any translations thereof into another language, the English language version shall govern.
16.8. Construction. This Agreement is the joint work product of representatives of each party. Accordingly, in the event of ambiguities in this Agreement, no inference will be drawn against either Party, including the Party that drafted this Agreement in its final form. The words "include," "includes," "including" and similar words shall are deemed to be followed by "without limitation" whether or not they are in fact followed by such words or words of like import. The words "herein" and "hereunder" and other words of similar import refer to this Agreement as a whole and not to any particular article, section or other subdivision. References in this Agreement to "provisions of this Agreement" refer to the terms, conditions and promises contained in this Agreement taken as a whole. Unless otherwise specified herein, all references to days, months, quarters or years are references to calendar days, calendar months, calendar quarters, or calendar years. References to the singular include the plural. All references to "$" or "dollars" are to United States Dollars. All references to an agreement or other instrument or statute or regulation are referred to as amended and supplemented from time to time and, in the case of a statute or regulation, any successor provisions thereof. Capitalized terms used in this Agreement shall have the meanings ascribed to them in the body of this Agreement or the applicable appendices. Terms other than those defined in this Agreement or the applicable appendices shall be given their plain English (or relevant alternative language, if in a language other than English) meaning and terms of art having a specialized meaning in the relevant industry shall be construed in accordance with industry standards. Unless the context otherwise requires, words importing the singular include the plural and vice-versa and words importing the masculine include the feminine and neuter and vice-versa.
| DARTMOUTH | CONFIDENTIAL |
16.9. Captions. The captions and headings used in this Agreement are for convenience only and do not limit or amplify the terms and provisions hereof.
16.10. Binding Effect; No Third Parties Beneficiaries. This Agreement shall be binding upon, and inure to the benefit of, the Parties and their respective successors and permitted assigns. Except as set forth herein, nothing in this Agreement is intended to confer on any person or entity, other than the parties and their respective successors and permitted assigns, any rights hereunder, except that Dartmouth will be a third party beneficiary of each Sublicense.
16.11. Severability. If any term or condition of this Agreement is determined to be invalid or unenforceable in whole or in part for any reason, this Agreement shall be reformed to be valid and enforceable consistent with the intention of the parties as expressed herein to the greatest extent permitted by law.
16.12. Counterparts; Validity of Electronic Copies; Amendments; Waivers. This Agreement may be executed in counterparts, each of which will be deemed an original, but all of which together shall constitute one and the same instrument. An executed copy of this Agreement that is delivered by facsimile or other electronic means shall be sufficient to show execution and delivery thereof. This Agreement may be amended only by a written document signed by authorized representatives of both parties. No waiver of any provision of this Agreement will be valid or binding unless set forth in writing and signed by the party granting the waiver. No failure by a party to exercise, and no delay by a party in exercising, any right hereunder will operate as a waiver of such right, nor will any single or partial exercise by a party of any right hereunder preclude any future exercise of that right, or any other right, by that party.
| DARTMOUTH | CONFIDENTIAL |
[Signature page follows]
| DARTMOUTH | CONFIDENTIAL |
IN WITNESS WHEREOF, Dartmouth and Licensee have executed this Agreement as of the Effective Date, in duplicate originals, by their respective and duly authorized officers.
| ALGERNON PHARMACEUTICALS INC. | TRUSTEES OF DARTMOUTH COLLEGE: |
| By: /s/ Christopher J. Moreau | By: /s/ Kim Rosenfield |
| Name: Christopher J. Moreau | Name: Kim Rosenfield |
| Title: CEO | Title: Director, Technology Transfer Office |
| Date: August 6, 2021 | Date: August 6, 2021 |
| DARTMOUTH | CONFIDENTIAL |
Appendix A
LICENSED PATENTS AND PATENT APPLICATIONS
1. US Patent 9,084,775 "Methods for diagnosing and treating neuroendocrine cancer".
| DARTMOUTH | CONFIDENTIAL |
Appendix B
COMMERCIALIZATION PLAN
