Hope for the Treatment of Kidney Fibrosis
Chronic Kidney Disease (CKD) is a condition in which the kidneys are damaged or cannot filter blood as well as healthy kidneys, often as a result of fibrosis. Algernon is in the early planning stages of its clinical program researching NP-251 (Repirinast) as a potential treatment for kidney inflammation and fibrosis.
Mechanism of Action
Mast cells are recruited to sites of cellular damage, and degranulation of mast cells leads to release of a myriad of proinflammatory chemical mediators which lead to tissue damage in a self-propagating cascade. NP-251 binds to receptors on mast cells and prevents their degranulation, which the Company believes could help prevent fibrosis in multiple organ classes including the kidneys.
Intellectual Property
Algernon’s patent applications for Repirinast include the treatment of CKD as well as dosing and in combination with several cholesterol-lowering and/or antihypertensive drugs.
About Chronic Kidney Disease
With CKD, including fibrosis, excess fluid and waste from the blood remain in the body and may cause other health problems. While there is no known cure, kidney disease complications can be controlled to make patients more comfortable. Treatments are focused on managing symptoms and complications that include high blood pressure, swelling and anemia.
NP-251 Development
Candidate / Indication
Development Stages
Pre-Clinical
Phase 1
Phase 2
Phase 3
Regulatory Review
Repirinast
Chronic Kidney DiseaseOriginally a Treatment for Asthma
Repirinast was originally developed by Mitsubishi Tanabe Pharma (“Mitsubishi”) and was sold and marketed in Japan under the brand name RometTM for the treatment of Asthma. RometTM was marketed for over 25 years in Japan. Mitsubishi discontinued manufacturing and sales of the drug in 2013. Accordingly, Algernon has retained Zhejiang Ausun Pharmaceutical in Zhejiang, China to manufacture a cGMP Repirinast supply.
Repirinast was approved in Japan for patients with bronchial asthma in 1987, to prevent attacks when administered regularly. A pediatric formulation was also approved in 1990. Unlike most allergy medications, Repirinast does not have a direct antihistaminic effect. The drug acts on mast cells and inhibits the release of chemical mediators by IgE-related antigen antibody interactions.
CKD Market
According to Transparency Market Research the global market for CKD drugs continues to grow at a significant pace, driven by the increasing number of CKD patients and the growing need for novel treatments to improve patients’ quality of life. The global CKD drug market stood at USD $11.5 Billion in 2015. Growing at a CAGR of 3.60% between 2016 and 2024, the market’s opportunity is expected to reach USD $15.8 Billion by the end of 2024.
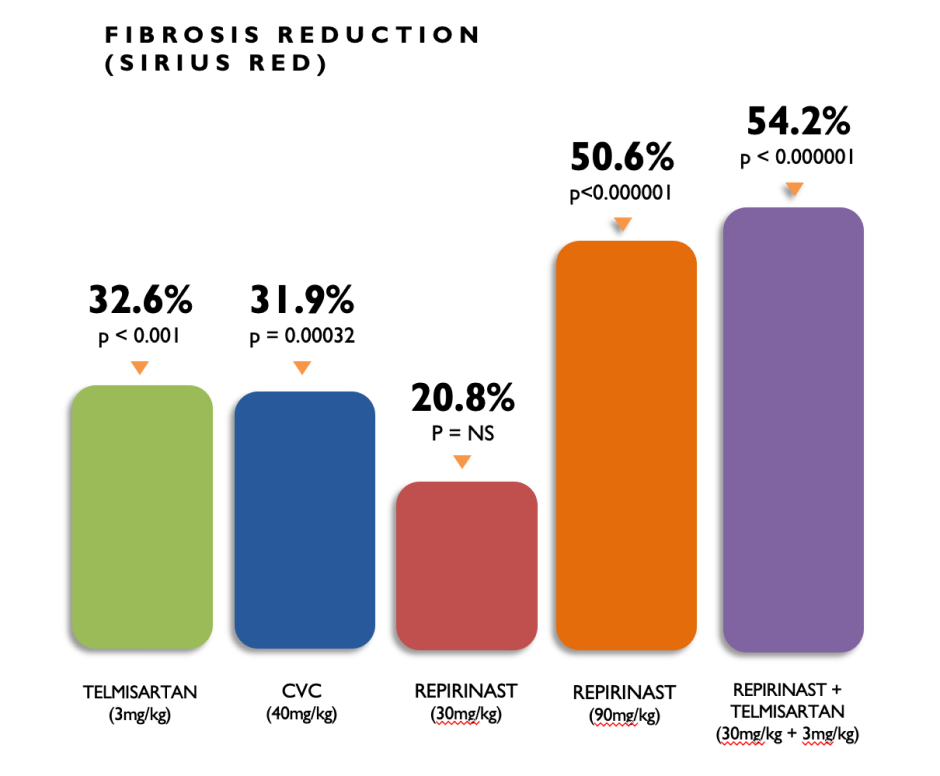
Preclinical Data for CKD
Repirinast, in a unilateral ureteral obstruction (UUO) mouse model of kidney fibrosis conducted by NASH Pharmaceuticals (an earlier Algernon acquisition), reduced fibrosis by 50% with statistical significance. It also showed a modest, but significant, synergistic improvement in combination with telmisartan, which is a blood pressure lowering medication and is considered a front-line standard of care treatment for CKD. As part of our CKD research program, we are also planning to investigate the use of Repirinast in acute interstitial nephritis.
Clinical Trials for CKD
We are in the early planning stages of our clinical research program for Repirinast and intend to file a pre-IND application with the U.S. Food and Drug Administration to conduct trials in the U.S. and intend to conduct our early clinical research programming in Australia. A Phase 1 trial is anticipated to begin in Q2 2026.

Q2, 2026
Conduct a small Phase 1 study to determine comparative pharmacokinetics of Repirinast in healthy volunteers and CKD patients.

Program Advisors
We have engaged several global experts in the area of chronic kidney disease research and have retained the following medical and scientific advisors.
Check back for new appointments.
Algernon in the Media
Discover the latest news and developments about our companies and research programs.
Read MoreAP-188 (DMT) - Stroke and Traumatic Brain Injury
Our AP-188 (DMT) candidate is based on a naturally occurring compound that has been shown in several preclinical studies to induce and increase neuroplasticity. We aim to repurpose DMT as a potential new treatment for stroke and traumatic brain injury (TBI) patients.
Learn More